1 Which of the Following Best Describes an Atom
B An atom has equal number of electrons and neutrons. One atom pulls an electron from another atom.
Forming bonds releases energy and is exothermic.

. Two atoms share an electron. It has a. Two atoms attain equal electronegativities.
At room temperature this element is a very stable gas. Which of the following statement is always correct. Which of the following best describes an electron.
C An atom has equal number of protons and neutrons. What are the fundamental particles of an atom. Learn about the fundamental particles also known as elementary particles and the types of particles.
D An atom has equal number of electrons protons and neutrons. Which of the following happens when an ionic bond is formed. A An atom has equal number of electrons and protons.
One atom becomes more electronegative than another atom. An atom of a certain element has 36 protons 36 electrons and a mass number of 84. - Which statement about bonding is correct.
It has no charge and about the same mass as a proton. - Get the answer to this question and access a vast question bank that is tailored for.

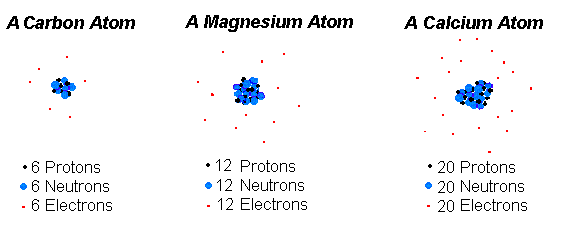
Labeled Parts Of An Atom Diagram Atom Diagram Atom Worksheets



Comments
Post a Comment